All Up Multi Wedge - pressure-relieving cushion
This product series and all belonging products have been discontinued by Levabo ApS since 20-01-2026
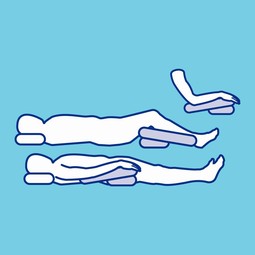
Wedge-shaped positioning cushion for pressure relief.
For seated and bedridden users.
Ensures even pressure distribution and supportive positioning.
Suitable for elevation of oedematous forearms and hands and for knee support.
Inflatable cushion with skin-friendly, moisture-absorbing nonwoven felt. (CE/MDR 2017)
For seated and bedridden users.
Ensures even pressure distribution and supportive positioning.
Suitable for elevation of oedematous forearms and hands and for knee support.
Inflatable cushion with skin-friendly, moisture-absorbing nonwoven felt. (CE/MDR 2017)
Classification
09 07 06 10 - Wedge-shaped positioning cushions
Documents
The product series includes 1 discontinued product.
Click Include discontinued products to show discontinued products and to see if the HMI-numbers in the product series are marketed by others.




 QuickGuide
QuickGuide