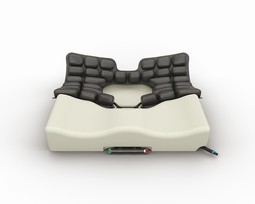
ROHO Hybrid Select
ROHO Hybrid Select is for users at risk of pressure ulcers and in need for positioning and stabilization of the pelvis and thighs. Consists of a shaped foam base with high density and a neoprene air insert with 3 chambers and ISOFLO. The mid part of the insert can be removed completely for complete relief of IT and tailbone. Complete with cover.
Classification
04 33 03 03 - Air cushions for pressure-sore prevention, static
Documents
The product series contains 22 products. The product below meets the specified details.
Click show all products to see all products belonging to the product series.
ROHO Hybrid Select w/HD cover 40,5x42,5cm
HMI-no.
131866
Article-no.
HS1616C
Registration date
04-02-2022
Last updated
17-10-2025
Properties
Can be adjusted asymmetrically
Yes
The cushion as a whole is machine washable
No
Measures
Width
40.5
cm
Height
13
cm
User weight, max
227
kg
Test information Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
EN 12182:2012: Assistive products for persons with disability - General requirements and test methods.
Test lab: Berlin Cert - Prüf- und Zertifizierstelle für Medizinprodukte GmbH,. Test date: 25-08-2021 Test report for HMI-no. 131866
Test report for HMI-no. 131866
Test lab: Berlin Cert - Prüf- und Zertifizierstelle für Medizinprodukte GmbH,. Test date: 25-08-2021
 Test report for HMI-no. 131866
Test report for HMI-no. 131866EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 131866
EU declaration of conformity for HMI-no. 131866