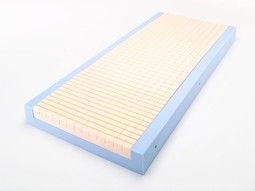
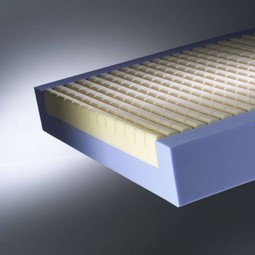
Softform Premier (former Superior)
Softform Premier: developed in partnership with clinicians. Serves as a high end pressure re-distributing mattr. for mittle to high risk patients and as suppl. to treatment of cat. 1 - 3 pressure ulcers. Practical and durable. High comfort level. Userweight: 0 - 247 kg. Special care for calf and heels.
Classification
04 33 06 13 - Foam mattresses, synthetic (PUR)
Documents
The product series contains 10 products.
The product series also includes 3 discontinued products. Include discontinued products. The product below meets the specified details.
Click show all products to see all products belonging to the product series.
Softform Premier, 80 x 200 x 15 cm
HMI-no.
42356
Article-no.
1486845
Registration date
07-02-2006
Last updated
05-02-2025
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
Yes
Measures
Length
200
cm
Width
80
cm
Height
15
cm
Mattress weight
13
kg
User weight, max
240
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device





 Brochure
Brochure