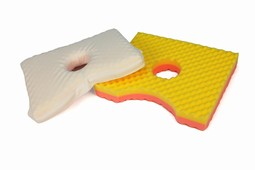
SAFE Med pressure relieving Ear Pillow
SAFE Med pressure relieving Ear Pillow prevents pressure ulcers and promotes healing supplying pain relief with comfortable pressure relief of the ear lying on the side.
BUY the very skin friendly Soft-Cell cover, Comfor PUR or Cotton velvet cover. See specifications below.
Machine wash up to 95 C, tumble dry.
Use with Yellow soft side up.
BUY the very skin friendly Soft-Cell cover, Comfor PUR or Cotton velvet cover. See specifications below.
Machine wash up to 95 C, tumble dry.
Use with Yellow soft side up.
Classification
09 07 06 05 - Positioning pillows and positioning cushions for head and neck
Documents
The product series contains 1 product. The product below meets the specified details.
SAFE Med pressure relieving Ear Pillow
HMI-no.
54575
Article-no.
115
Registration date
05-02-2010
Last updated
29-07-2025
Properties
Shape
Rektangulær
Filling
Skum
(Optional) machine washable cover
Yes
(Optional) cover which can be autoclaved
Yes
(Optional) cover which can be disinfected
Yes
Machine washable cushion
Yes
Cushion can be autoclaved
Yes
Cushion can be tumble dried
Yes
Flame resistant
Yes
Measures
Height
6
cm
Longest side
40
cm
Shortest side
32
cm
Test information Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Swerea IVF AB. Test date: 07-11-2018 Test report for HMI-no. 54575
Test report for HMI-no. 54575
Test lab: Swerea IVF AB. Test date: 07-11-2018
 Test report for HMI-no. 54575
Test report for HMI-no. 54575EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Other specifications
Dimension: 40 x 32 x 6 cm