SAFE Med OP-helmadras til intensivpatienter
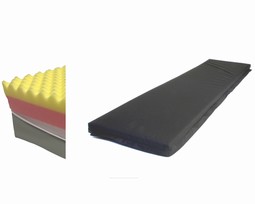
SAFE Med OP-fullbody mattress for intensive care assures pressure relief, stability and is pain relieving. User weight is for thin, normal weight and moderat overweight up to 130 kg or BMI up to 35. Mattress has a carrying capacity up to 180 kg. For user up to stage 3. The cover fulfills all demands to desinfect and clean.
Classification
04 33 06 13 - Foam mattresses, synthetic (PUR)
Documents
The product series contains 3 products. The product below meets the specified details.
Click show all products to see all products belonging to the product series.
SAFE Med OP-Fullbody mattress for intensive care, 200x90x12 cm
HMI-no.
63482
Article-no.
201YPOP.2
Registration date
08-03-2011
Last updated
05-02-2025
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
90
cm
Height
13
cm
Mattress weight
14
kg
User weight, max
130
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device



 Brochure
Brochure