All product information is provided by the supplier. The National Board of Social Services is not responsible for either contents, origin, flaws and deficiencies, or any kind of damage that may occur from the use of the information. The National Board of Social Services has no authority to endorse products and does not assess the quality of the products. Hide this message.
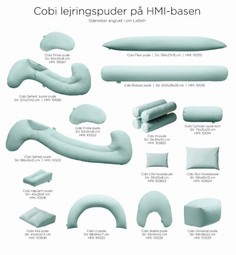
Cobi positioning Cushions
The cushions are primarily designed for neurologically injured patients and demented but both patients with much pain and as well as terminal patients benefit equally positive of the cushions. The focus has been on the usual positioning techniques and on flexibility and comfort.
The cushions retain its shape and ensures stable positioning.
The cushions retain its shape and ensures stable positioning.
Classification
09 07 06 14 - Other positioning cushions for general purposes
The product series contains 11 products.
The product series also includes a discontinued product. Include discontinued products. The product below meets the specified details.
Click show all products to see all products belonging to the product series.
Cobi H Cushion
HMI-no.
103835
Article-no.
0400-074-000
Registration date
08-02-2017
Last updated
05-05-2025
Properties
Filling
Granulat/kugler
(Optional) machine washable cover
Yes
(Optional) cover which can be autoclaved
No
(Optional) cover which can be disinfected
Yes
Machine washable cushion
No
Cushion can be autoclaved
No
Cushion can be tumble dried
No
Measures
Longest side
51
cm
Shortest side
30
cm
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
: Tested according to other national or international standard.
Test lab: Centexbel. Test date: 27-05-2021 Test report for HMI-no. 103835
Test report for HMI-no. 103835
Test lab: Centexbel. Test date: 27-05-2021
 Test report for HMI-no. 103835
Test report for HMI-no. 103835EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device