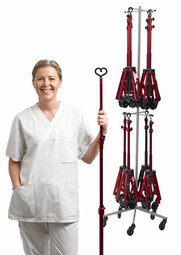
Hytech foldable IV stand
Classification
24 36 03 13 - Free standing drip stands
The product series includes 2 products of which 1 product is discontinued.
Product 1 of 2
Hytech foldable IV stand
HMI-no.
86177
Article-no.
08-3500-101
Registration date
06-12-2014
Last updated
12-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 86177
EU declaration of conformity for HMI-no. 86177Product 2 of 2
Hytech foldable IV stand
The product was discontinued from Hytech Medico 17-08-2020HMI-no.
86178
Article-no.
Hytech dropstativ 2 delt
Registration date
06-12-2014
Last updated
17-08-2020
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation
No information about CE-marking The supplier has not provided any information about CE-marking of the product. Explain CE-marking