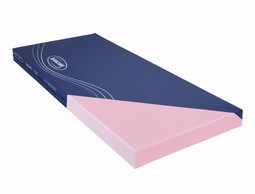
Dacapo Basic foam mattress
Dacapo Basic - is the first mattress
in the Dacapo series. It fully satisfies basic comfort &
hygiene requirements for a sleeping surface. The
high density, high resilient, foam provides a firm
supportive base, strength and durability.
in the Dacapo series. It fully satisfies basic comfort &
hygiene requirements for a sleeping surface. The
high density, high resilient, foam provides a firm
supportive base, strength and durability.
Classification
04 33 06 13 - Foam mattresses, synthetic (PUR)
Documents
The product series contains 1 product.
The product series also includes 7 discontinued products. Include discontinued products.
Product 1 of 1
Dacapo Basic Special 200x83x5
HMI-no.
47512
Article-no.
60131001
Registration date
17-12-2007
Last updated
05-02-2025
Properties
Intended for use with adjustable mattress support platform
Yes
Fire-retardant foam
No
Measures
Length
200
cm
Width
83
cm
Height
5
cm
Mattress weight
5
kg
User weight, max
130
kg
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Other specifications
Dacapo Basic Special 200x83x5




 Brochure
Brochure