All product information is provided by the supplier. The National Board of Social Services is not responsible for either contents, origin, flaws and deficiencies, or any kind of damage that may occur from the use of the information. The National Board of Social Services has no authority to endorse products and does not assess the quality of the products. Hide this message.
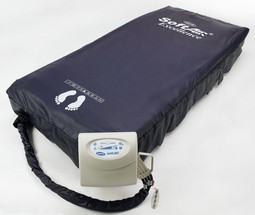
Invacare SoftAir Super
This product series and all belonging products have been discontinued by Invacare A/S since 13-01-2021
The Invacare SoftAIR dynamic mattresses are designed for patients
at Very High Risk of developing pressure ulcers. There are two
options available within the SoftAIR range, The SoftAIR Super,
and the SoftAIR Excellence.
at Very High Risk of developing pressure ulcers. There are two
options available within the SoftAIR range, The SoftAIR Super,
and the SoftAIR Excellence.
Classification
04 33 06 16 - Air mattresses, dynamic
Documents
The product serie includes 2 products. 2 of these products are discontinued from the supplier.
Product 1 of 2
Invacare SoftAir Super 83 x 200 x 20 cm
The product was discontinued from Invacare A/S 13-01-2021HMI-no.
54706
Article-no.
1532815
Registration date
24-02-2010
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
Combination mattress
No

Air mattress filled with both air and other material e.g. foam or water
Divided into sections
Yes

Divided into sections with differing construction with respect to various parts of the body.
Rapid mattress deflation
Yes
Built in lateral tilt function
No
Can be used with underlying powered turning systems
Yes
Can be placed directly on mattress support platform
Yes
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
83
cm

For mattress extension: equivalent to the widht of the mattress.
Height
20
cm
Mattress weight
8.4
kg
User weight, max
200
kg
Test information
No information about tests according to standards 

The supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
CE marked according to other specific regulations/directives (e.g. machinery, construction products, low voltage)
CE marked according to other specific regulations/directives (e.g. machinery, construction products, low voltage)
Other specifications
User weight 200 kg. Size 200 x 83 x 20 cm. Cell-in-Cell design
Product 2 of 2
Invacare SoftAir Super 88 x 200 x 20 cm
The product was discontinued from Invacare A/S 13-01-2021HMI-no.
54707
Article-no.
1532816
Registration date
24-02-2010
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
Combination mattress
No

Air mattress filled with both air and other material e.g. foam or water
Divided into sections
Yes

Divided into sections with differing construction with respect to various parts of the body.
Rapid mattress deflation
Yes
Built in lateral tilt function
No
Can be used with underlying powered turning systems
Yes
Can be placed directly on mattress support platform
Yes
Intended for use with adjustable mattress support platform
Yes
Measures
Length
200
cm

For mattress extension: measure of the elongation of the mattress.
Width
88
cm

For mattress extension: equivalent to the widht of the mattress.
Height
20
cm
Mattress weight
8.4
kg
User weight, max
200
kg
Test information
No information about tests according to standards 

The supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
CE marked according to other specific regulations/directives (e.g. machinery, construction products, low voltage)
CE marked according to other specific regulations/directives (e.g. machinery, construction products, low voltage)
Other specifications
User weight 200 kg. Size 200 x 88 x 20 cm. Cell-in-Cell design






 Brochure
Brochure