Apodan Lympha Press Comfy Benmanchet, multikammer
Classification
04 06 09 04 - Air-filled attachments and pulsating compression systems, leg
Documents
The product series contains 7 products.
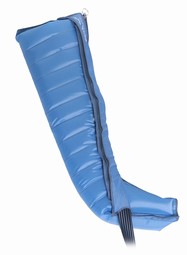
Product 1 of 7
Lympha Press Comfy Benmanchet med lynlås, multichamber, str. 4-75
HMI-no.
103362
Article-no.
022194
Registration date
04-01-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 7
Lympha Press Comfy Benmanchet med lynlås, multikammer, str. 2-75
HMI-no.
109729
Article-no.
022195
Registration date
22-05-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 7
Lympha Press Comfy Legsleeve with zipper, multichamber, str. 2-85
HMI-no.
65091
Article-no.
022189
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 4 of 7
Lympha Press Comfy Legsleeve with zipper, multichamber, str. 2-95
HMI-no.
65092
Article-no.
022190
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 5 of 7
Lympha Press Comfy Legsleeve with zipper, multichamber, str. 3-65
HMI-no.
65118
Article-no.
022191
Registration date
13-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 6 of 7
Lympha Press Comfy Legsleeve with zipper, multichamber, str. 3-75
HMI-no.
65093
Article-no.
022192
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 7 of 7
Lympha Press Comfy Legsleeve with zipper, multichamber, str. 3-85
HMI-no.
65094
Article-no.
022193
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device






 Brochure
Brochure