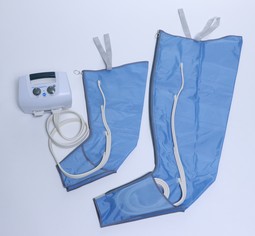
Apodan Lympha Press Benmanchet, 4 luftkamre
Classification
04 06 09 04 - Air-filled attachments and pulsating compression systems, leg
Documents
The product series contains 10 products.
The product series also includes 10 discontinued products. Include discontinued products.
Product 1 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 2-50
HMI-no.
107380
Article-no.
024050
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 2-75
HMI-no.
109947
Article-no.
024045
Registration date
14-06-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 2-85
HMI-no.
113560
Article-no.
024044
Registration date
18-12-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 4 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 2-95
HMI-no.
113561
Article-no.
024043
Registration date
18-12-2018
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 5 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 3-65
HMI-no.
107381
Article-no.
024051
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 6 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 3-75
HMI-no.
106944
Article-no.
024052
Registration date
13-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 7 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 3-85
HMI-no.
107346
Article-no.
024053
Registration date
23-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 8 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 4-65
HMI-no.
107382
Article-no.
024046
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 9 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 4-75
HMI-no.
107383
Article-no.
024047
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 10 of 10
Lympha Press Benmanchet, 4 luftkamre, str. 4-85
HMI-no.
107384
Article-no.
024048
Registration date
27-11-2017
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device






 Brochure
Brochure