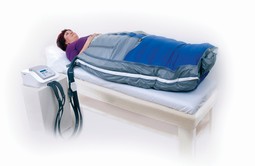
Apodan Lympha Press LymphaPod Buksemanchet, 150-300 kg
Classification
04 06 09 04 - Air-filled attachments and pulsating compression systems, leg
Documents
The product series contains 3 products.
Product 1 of 3
Lympha Press, LymphaPod manchet, 150-300 kg
HMI-no.
146919
Article-no.
022178
Registration date
07-01-2026
Last updated
07-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146919
EU declaration of conformity for HMI-no. 146919Other specifications
Hose on the left side.
Open at the foot end.
Open at the foot end.
Product 2 of 3
Lympha Press, LymphaPod manchet, 150-300 kg
HMI-no.
65113
Article-no.
022179
Registration date
12-09-2011
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 3
Lympha Press, LymphaPod manchet, 150-300 kg
HMI-no.
78892
Article-no.
022181
Registration date
08-05-2013
Last updated
24-03-2022
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device