Bandage
Classification
06 12 13 01 - Lower leg orthoses
Documents
The product series contains 10 products.
Product 1 of 10
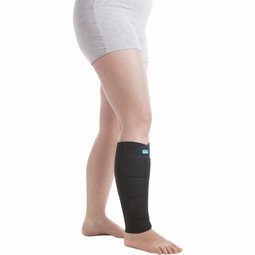
Bandage, underbensegment, sort, 1-xsmall-lang-højre
HMI-no.
107454
Article-no.
1000003270
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 10
Bandage, underbensegment, sort, 1-xsmall-lang-venstre
HMI-no.
107455
Article-no.
1000003265
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 10
Bandage, underbensegment, sort, 1-xsmall-normal-højre
HMI-no.
107452
Article-no.
1000003275
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 4 of 10
Bandage, underbensegment, sort, 1-xsmall-normal-venstre
HMI-no.
107459
Article-no.
1000001709
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 5 of 10
Bandage, underbensegment, sort, 2-small-normal-venstre
HMI-no.
107438
Article-no.
1000001710
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 6 of 10
Bandage, underbensegment, sort, 3-medium-normal-højre
HMI-no.
107451
Article-no.
1000003277
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 7 of 10
Bandage, underbensegment, sort, 3-medium-normal-venstre
HMI-no.
107466
Article-no.
1000001711
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 8 of 10
Bandage, underbensegment, sort, 4-large-normal-højre
HMI-no.
107450
Article-no.
1000003278
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 9 of 10
Bandage, underbensegment, sort, 4-large-normal-venstre
HMI-no.
107465
Article-no.
1000001712
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 10 of 10
Bandage, underbensegment, sort, 5-xlarge-lang-højre
HMI-no.
107453
Article-no.
1000003274
Registration date
28-11-2017
Last updated
06-11-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device



 Brochure
Brochure