Bord til Delfi Pro
Classification
12 25 24 01 - Lap trays for wheelchairs
Documents
The product series contains 3 products.
Product 1 of 3
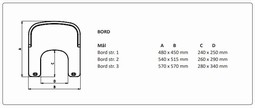
Delfi Pro, Bord str. 1, transparent
HMI-no.
115012
Article-no.
990251
Registration date
06-03-2019
Last updated
10-11-2024
Properties
Raised edge
Yes
Allows entry and exit without disassembling
No
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 2 of 3
Delfi Pro, Bord str. 2, transparent
HMI-no.
115013
Article-no.
990252
Registration date
06-03-2019
Last updated
10-11-2024
Properties
Raised edge
Yes
Allows entry and exit without disassembling
No
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Product 3 of 3
Delfi Pro, Bord str. 3, transparent
HMI-no.
115014
Article-no.
990253
Registration date
06-03-2019
Last updated
10-11-2024
Properties
Raised edge
Yes
Allows entry and exit without disassembling
No
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device




 Brochure
Brochure