All product information is provided by the supplier. The National Board of Social Services is not responsible for either contents, origin, flaws and deficiencies, or any kind of damage that may occur from the use of the information. The National Board of Social Services has no authority to endorse products and does not assess the quality of the products. Hide this message.
ROHO Hybrid Select
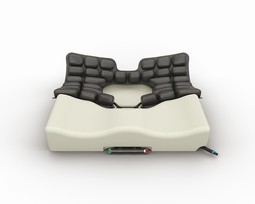
ROHO Hybrid Select is for users at risk of pressure ulcers and in need for positioning and stabilization of the pelvis and thighs. Consists of a shaped foam base with high density and a neoprene air insert with 3 chambers and ISOFLO. The mid part of the insert can be removed completely for complete relief of IT and tailbone. Complete with cover.
Classification
04 33 03 03 - Air cushions for pressure-sore prevention, static
Documents
The product series contains 22 products. The product below meets the specified details.
Click show all products to see all products belonging to the product series.
ROHO Hybrid Select w/HD cover 40,5x40cm
HMI-no.
131867
Article-no.
HS1615C
Registration date
04-02-2022
Last updated
21-11-2023
Properties
Intended for children
No

Products for children must comply with the specific demands for safety as stated in certain standards. It is the supplier of the product who has stated that the product is intended for children. The National Board of Social Services holds no responsibility in relation to this assessment.
Can be adjusted asymmetrically
Yes
The cushion as a whole is machine washable
No
Measures
Width
40.5
cm
Depth
40
cm

Corresponding to the seat depth of the chair
Height
13
cm
Weight
1450
g
User weight, max
227
kg
Test information 

Though the product is testet, it might not have passed all requirements in the standard. Besides, some products are only tested according to parts of the standard. Read the test report for detailed information. See also other standards that could be relevant for this product type.
EN 12182:2012: Assistive products for persons with disability - General requirements and test methods.
Test lab: Berlin Cert - Prüf- und Zertifizierstelle für Medizinprodukte GmbH,. Test date: 25-08-2021 Test report for HMI-no. 131867
Test report for HMI-no. 131867
Test lab: Berlin Cert - Prüf- und Zertifizierstelle für Medizinprodukte GmbH,. Test date: 25-08-2021
 Test report for HMI-no. 131867
Test report for HMI-no. 131867EU product safety regulation 

The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device