Autodrop - bottle holder for eye drops
Classification
04 19 33 01 - Other assistive products for administering medicines
Documents
The product series contains 1 product.
Product 1 of 1
Autodrop - bottle holder for eye drops
HMI-no.
Article-no.
620058
Registration date
30-12-2022
Last updated
14-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
Exclusively within the general product safety regulation/directive and without CE marking




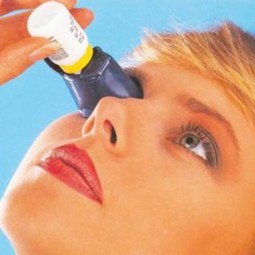
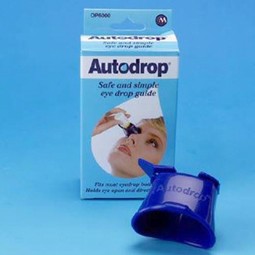
 User manual
User manual