GyroGloveTM
GyroGloveTM harnesses the power of advanced gyroscopic stabilization to counteract the debilitating effects of hand tremors, giving the user back the control that had been lost to tremor. The result is a steady hand.
Classification
06 06 12 01 - Wrist-hand orthoses
The product series contains 1 product.
Product 1 of 1
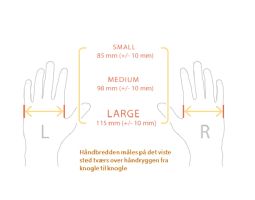
GyroGlove Reduces Tremor In Your Hand
HMI-no.
138099
Article-no.
3-176-001
Registration date
05-09-2023
Last updated
13-08-2024
Properties
With action on the wrist
No
With action on the base of the thumb
No
With baleens
No
Elastic material
Yes
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 138099
EU declaration of conformity for HMI-no. 138099