4-point knee braces
For indications as
Osteoarthritis/valgus/varus (OA)
Post-traumatic/postoperative (OA, CI, GENU-Tex)
Pain relief in joint chambers (OA)
Partial/reconstructed ACL/PCL (CI, GENU-Tex)
Ligament rupture/instability (CI, GENU-Tex)
Drawer laxity in the knee (GENU-Tex)
Meniscus reconstruction (GENU-Tex)
Collateral ligament insufficiency (GENU-Tex)
Osteoarthritis/valgus/varus (OA)
Post-traumatic/postoperative (OA, CI, GENU-Tex)
Pain relief in joint chambers (OA)
Partial/reconstructed ACL/PCL (CI, GENU-Tex)
Ligament rupture/instability (CI, GENU-Tex)
Drawer laxity in the knee (GENU-Tex)
Meniscus reconstruction (GENU-Tex)
Collateral ligament insufficiency (GENU-Tex)
Classification
06 12 09 01 - Knee orthoses
Documents
Video
The product series contains 3 products.
Product 1 of 3
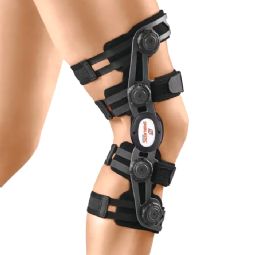
4-point knee brace - GENUDYN CI
HMI-no.
139303
Article-no.
7780
Registration date
11-12-2023
Last updated
24-09-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Other specifications
Indications:
ACL/PCL lesions
Partial ligament rupture
Reconstructed ACL/PCL
Drawer laxity in knee
Collateral ligament rupture/insufficiency
Instability
Post-traumatic/postoperative
Severe instability
ACL/PCL lesions
Partial ligament rupture
Reconstructed ACL/PCL
Drawer laxity in knee
Collateral ligament rupture/insufficiency
Instability
Post-traumatic/postoperative
Severe instability
Product 2 of 3
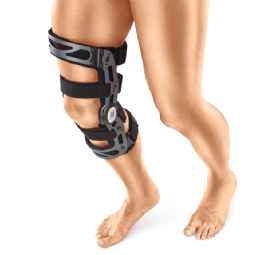
4-point knee brace - GENU-TEX
HMI-no.
139304
Article-no.
7720
Registration date
11-12-2023
Last updated
24-09-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Other specifications
Indications:
Instability
Osteoarthritis
Rheumatoid arthritis/RA
ACL rupture
Partial rupture
Reconstructed ACL
Drawer laxity in the knee
Meniscus reconstruction
Ligament rupture/insufficiency of collateral ligaments
Instability
Osteoarthritis
Rheumatoid arthritis/RA
ACL rupture
Partial rupture
Reconstructed ACL
Drawer laxity in the knee
Meniscus reconstruction
Ligament rupture/insufficiency of collateral ligaments
Product 3 of 3
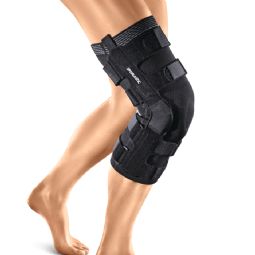
4-point knee brace GENUDYN CI NOVEL
HMI-no.
139277
Article-no.
7785
Registration date
04-12-2023
Last updated
24-09-2024
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
Other specifications
Indications:
ACL and/or PCL rupture
Partial ruptures
Reconstructed ACL and/or PCL
Drawer laxity in the knee
Ligament rupture/insufficiency of collateral ligaments
Instability
Post-traumatic
Postoperative
Severe instability
ACL and/or PCL rupture
Partial ruptures
Reconstructed ACL and/or PCL
Drawer laxity in the knee
Ligament rupture/insufficiency of collateral ligaments
Instability
Post-traumatic
Postoperative
Severe instability



 Brochure
Brochure Play video
Play video