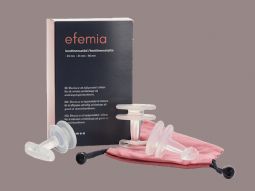
efemia
Classification
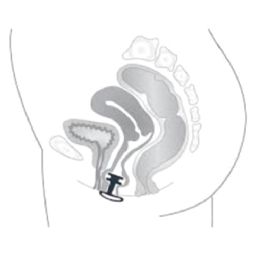
09 31 03 01 - Vaginal devices for compression of the urethra
Documents
The product series contains 3 products.
Product 1 of 3
efemia size 1
HMI-no.
143909
Registration date
04-03-2025
Last updated
17-03-2025
Properties
Material
Silikone
Measures
Recommended usage time
16
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 143909
EU declaration of conformity for HMI-no. 143909Product 2 of 3
efemia size 2
HMI-no.
143912
Registration date
04-03-2025
Last updated
17-03-2025
Properties
Material
Silikone
Measures
Recommended usage time
16
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 143912
EU declaration of conformity for HMI-no. 143912Product 3 of 3
efemia size 3
HMI-no.
143915
Registration date
04-03-2025
Last updated
17-03-2025
Properties
Material
Silikone
Measures
Recommended usage time
16
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 143915
EU declaration of conformity for HMI-no. 143915