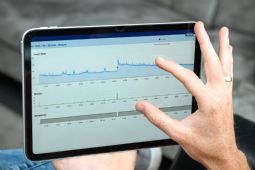
NightWatch Plus - Pro
Monitoring system for hospitals and care homes. Tracks heart rate, movement and body position. Detects tonic-clonic, tonic (in clusters/prolonged), myoclonic (in clusters) and hyperkinetic seizures. Sends alerts to an external call system.
Classification
22 29 06 01 - Seizure-alarms for persons with epilepsy
The product series contains 1 product.
Product 1 of 1
NightWatch Plus - Pro
HMI-no.
144864
Article-no.
NW003
Registration date
10-06-2025
Last updated
10-06-2025
Properties
Automatic low battery warning
Yes
Portable sensor
Yes
Adjustment of sensitiveness
No
Adjustment of time
No
Programmable customization
No
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 144864
EU declaration of conformity for HMI-no. 144864