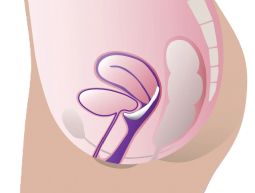
ProFem Marland Pessary
With or without a support membrane, it is used to treat symptoms of second- or third-degree uterine prolapse and cystocele. The raised ring may also help relieve stress incontinence. Holes in the membrane ease removal by releasing vacuum. Starting with a fitting set is recommended.
Classification
09 31 03 01 - Vaginal devices for compression of the urethra
Documents
The product series contains 14 products.
Product 1 of 14
ProFem Marland size 2, 58 mm
HMI-no.
145790
Article-no.
M2.25-2
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145790
EU declaration of conformity for HMI-no. 145790Product 2 of 14
ProFem Marland size 3, 64 mm
HMI-no.
145793
Article-no.
M2.50-3
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145793
EU declaration of conformity for HMI-no. 145793Product 3 of 14
ProFem Marland size 4, 70 mm
HMI-no.
145796
Article-no.
M2.75-4
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145796
EU declaration of conformity for HMI-no. 145796Product 4 of 14
ProFem Marland size 5, 76 mm
HMI-no.
145799
Article-no.
M3.00-5
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145799
EU declaration of conformity for HMI-no. 145799Product 5 of 14
ProFem Marland size 6, 83 mm
HMI-no.
145802
Article-no.
M3.25-6
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145802
EU declaration of conformity for HMI-no. 145802Product 6 of 14
ProFem Marland size 7, 89 mm
HMI-no.
145805
Article-no.
M3.50-7
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145805
EU declaration of conformity for HMI-no. 145805Product 7 of 14
ProFem Marland size 8, 95 mm
HMI-no.
145808
Article-no.
M3.75-8
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145808
EU declaration of conformity for HMI-no. 145808Product 8 of 14
ProFem Marland with support size 2, 58 mm
HMI-no.
145811
Article-no.
M2.25S-2
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145811
EU declaration of conformity for HMI-no. 145811Product 9 of 14
ProFem Marland with support size 3, 64 mm
HMI-no.
145814
Article-no.
M2.50S-3
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145814
EU declaration of conformity for HMI-no. 145814Product 10 of 14
ProFem Marland with support size 4, 70 mm
HMI-no.
145817
Article-no.
M2.75S-4
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145817
EU declaration of conformity for HMI-no. 145817Product 11 of 14
ProFem Marland with support size 5, 76 mm
HMI-no.
145820
Article-no.
M3.00S-5
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145820
EU declaration of conformity for HMI-no. 145820Product 12 of 14
ProFem Marland with support size 6, 83 mm
HMI-no.
145823
Article-no.
M3.25S-6
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145823
EU declaration of conformity for HMI-no. 145823Product 13 of 14
ProFem Marland with support size 7, 89 mm
HMI-no.
145826
Article-no.
M3.50S-7
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145826
EU declaration of conformity for HMI-no. 145826Product 14 of 14
ProFem Marland with support size 8, 95 mm
HMI-no.
145829
Article-no.
M3.75S-8
Registration date
05-09-2025
Last updated
16-09-2025
Properties
Material
Silikone
Measures
Recommended usage time
24
timer
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 145829
EU declaration of conformity for HMI-no. 145829