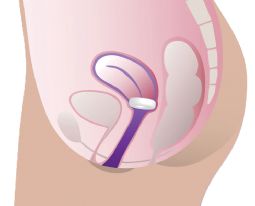
ProFem Donut Pessary
Soft donutshaped support pessary for treating symptoms of uterine prolapse grade 23, cystocele, and rectocele. Its round shape and soft material provide gentle pressure. The pessary can be compressed for easier insertion and removal. Especially suitable for postmenopausal women.
Classification
06 04 03 01 - Abdominal muscle supports
Documents
The product series contains 7 products.
Product 1 of 7
ProFem Donut Pessary size 0 - 50 mm
HMI-no.
146420
Article-no.
D2.00-0
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146420
EU declaration of conformity for HMI-no. 146420Product 2 of 7
ProFem Donut Pessary size 1 - 58 mm
HMI-no.
146423
Article-no.
D2.25-1
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146423
EU declaration of conformity for HMI-no. 146423Product 3 of 7
ProFem Donut Pessary size 2 - 64 mm
HMI-no.
146426
Article-no.
D2.50-2
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146426
EU declaration of conformity for HMI-no. 146426Product 4 of 7
ProFem Donut Pessary size 3 - 70 mm
HMI-no.
146429
Article-no.
D2.75-3
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146429
EU declaration of conformity for HMI-no. 146429Product 5 of 7
ProFem Donut Pessary size 4 - 76 mm
HMI-no.
146432
Article-no.
D3.00-4
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146432
EU declaration of conformity for HMI-no. 146432Product 6 of 7
ProFem Donut Pessary size 5 - 83 mm
HMI-no.
146435
Article-no.
D3.25-5
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146435
EU declaration of conformity for HMI-no. 146435Product 7 of 7
ProFem Donut Pessary size 6 - 88 mm
HMI-no.
146438
Article-no.
D3.50-6
Registration date
07-11-2025
Last updated
29-01-2026
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 146438
EU declaration of conformity for HMI-no. 146438