NightWatch EPI-Alarm
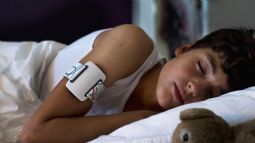
NightWatch is an epilepsy alarm worn on the upper arm by the user during sleep. It monitors heart rate, movement and body position to alert carers of severe nocturnal motor seizures. NightWatch is clinically validated and helps reduce seizure-related risks such as injury, status epilepticus and SUDEP
Classification
22 29 06 01 - Seizure-alarms for persons with epilepsy
Documents
Video
The product series contains 1 product.
Product 1 of 1
NightWatch EPI-Alarm
HMI-no.
143051
Article-no.
NW001
Registration date
16-01-2025
Last updated
02-06-2025
Properties
Automatic low battery warning
Yes
Portable sensor
Yes
Adjustment of sensitiveness
No
Adjustment of time
No
Programmable customization
No
Test information
No information about tests according to standardsThe supplier has not provided documentation, that this product is testet according to a relevant standard. See examples of relevant standards for this product type
EU product safety regulation The supplier has provided the following information about CE-marking of the product. Explain CE-marking
CE marked as medical device
 EU declaration of conformity for HMI-no. 143051
EU declaration of conformity for HMI-no. 143051Other specifications
Adjustment of sensitivity, time and customization is automatic during use.
Recommended and tested for people aged 4 years and older. If the child is younger, expect more false alarms. for people aged 4 years and older.
Recommended and tested for people aged 4 years and older. If the child is younger, expect more false alarms. for people aged 4 years and older.





 Play video
Play video